Single Plane Illumination Microscope (SPIM)
Jul 10, 2024
BCA is pleased to announce our next Spotlight Series on July 10 at 5pm EDT. Professor Ted Kinsman from Rochester Institute of Technology talk about Single Plane Illumination Microscopy (SPIM). If you missed it live we have now uploaded it to the BCA YouTube so you can still see this presentation.
Introduction to SPIM
In the realm of modern microscopy, the quest for clearer, faster, and less invasive imaging techniques has led to the development of various innovative methods. Among these, Single Plane Illumination Microscopy (SPIM) has emerged as a powerful tool for three-dimensional (3D - volumetric) imaging of biological specimens with exceptional clarity and minimal photodamage.
SPIM, also known as light sheet fluorescence microscopy, revolutionizes traditional imaging approaches by illuminating the specimen with a thin plane of light perpendicular to the observation axis. This distinctive illumination method enables selective illumination of the focal plane of interest, drastically reducing photobleaching compared to conventional fluorescence microscopy.
The key principle underlying SPIM lies in the decoupling of the illumination and detection pathways. By employing a separate light sheet for illumination and a perpendicular detection path, SPIM minimizes out-of-focus light, thus enhancing image contrast and resolution. This strategy not only mitigates phototoxicity but also allows for rapid image acquisition with minimal disturbance to delicate biological samples, making SPIM particularly well-suited for live-cell imaging and developmental biology studies.
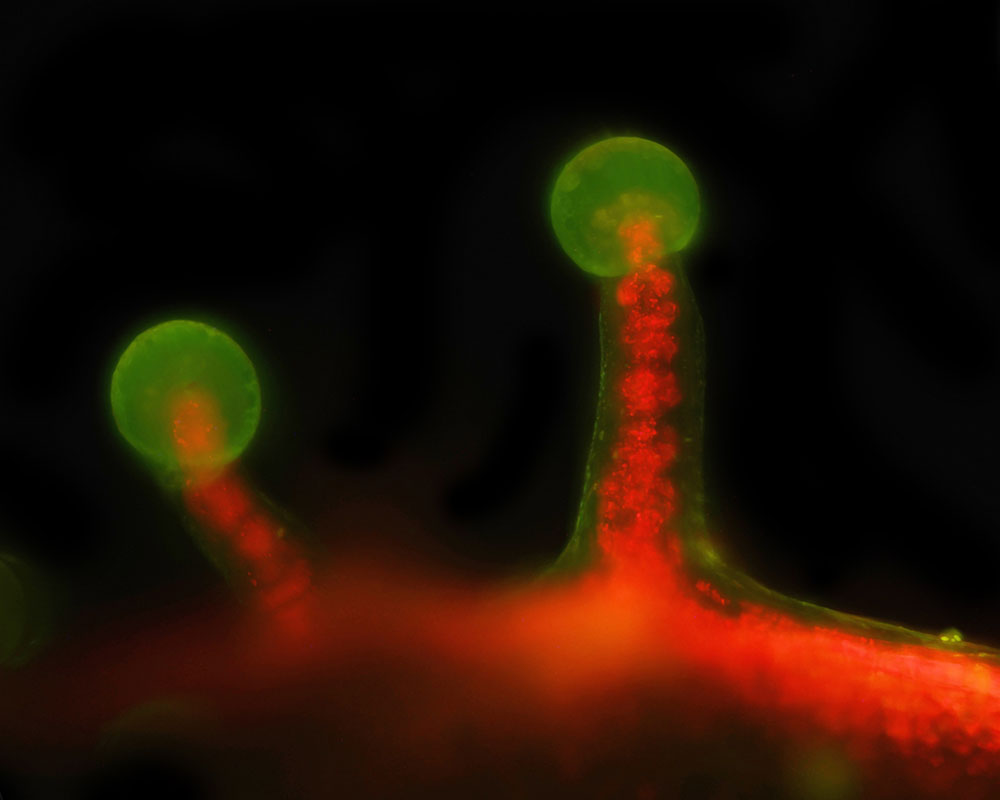
The specimen is auto-fluorescing and is bathed in a sheet of 405 nm laser light and is immersed in distilled water. Using a high numerical aperture (N.A.) water immersion lens, only the material in perfect focus is illuminated and thus imaged. A typical dataset involves the collection of hundreds of images, each at a different position of the specimen. The resulting images can be combined in 3D visualization software like FIJI to make a movie, or the stacks can be processed to create a 2D image by using various algorithms to highlight details of interest.
In addition to its benefits in reducing photodamage, SPIM excels in generating high-resolution 3D reconstructions of large, optically thick specimens like sphagnum moss, which is auto fluorescent due to the abundance of chlorophyll. By sequentially capturing optical sections at different depths and computationally stitching them together, SPIM provides detailed insights into the spatial organization and dynamics of biological structures in their native environment.
This project and design were built upon the framework developed by the Open SPIM project (https://openspim.org/). My design is modified for student use and incorporates an inexpensive laser pointer as the light source. A 4K Sony digital camera is the sensor, and 3D printing is used for the water immersion cell. Expensive 20X Olympus water immersion optics are compared to less expensive optics; the design was modified to take advantage of surplus optics.
Shown here are several examples from the microscope. The images were all collected at 20x with a water immersion objective.

 Ted Kinsman is a Professor of Photographic Sciences at Rochester Institute of Technology (RIT) where he teaches technical imaging. Ted presented on RTI at the 2022 BCA Symposium and at the Forensic Photography Symposium in 2023. Learn more about Ted here:
Ted Kinsman is a Professor of Photographic Sciences at Rochester Institute of Technology (RIT) where he teaches technical imaging. Ted presented on RTI at the 2022 BCA Symposium and at the Forensic Photography Symposium in 2023. Learn more about Ted here:
Instagram
https://www.rit.edu/directory/emkpph-ted-kinsman
